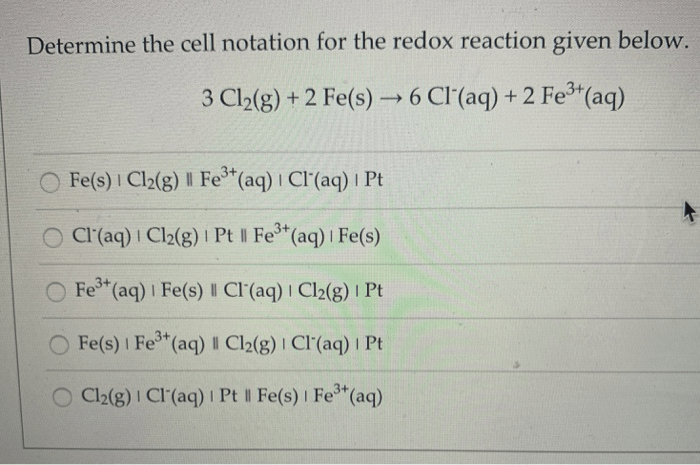Determine the cell notation for the redox reaction given below. Electrochemistry is the study of the relationship between electrical energy and chemical change. One important aspect of electrochemistry is the use of cell notation to represent electrochemical reactions. Cell notation provides a concise and informative way to describe the components and behavior of an electrochemical cell.
In this guide, we will explore the concept of cell notation, including how to identify half-reactions, balance them, and construct cell notation from balanced half-reactions. We will also provide practice problems to help you apply these concepts.
Define Cell Notation

Cell notation is a concise representation of an electrochemical cell, providing information about the chemical species involved, their oxidation states, and the direction of electron flow. It allows for a quick and comprehensive understanding of the electrochemical process.
Identifying Half-Reactions
To determine cell notation, it’s essential to identify the half-reactions involved in the redox reaction. Oxidation is the loss of electrons, while reduction is the gain of electrons. Identifying the half-reactions helps establish the anode (oxidation) and cathode (reduction) half-cells.
Balancing Half-Reactions, Determine the cell notation for the redox reaction given below
Balancing half-reactions ensures the conservation of mass and charge in the electrochemical cell. In acidic solutions, H+ ions and H2O molecules are involved, while in basic solutions, OH- ions and H2O molecules participate. Balancing involves adjusting the coefficients of species to ensure the number of electrons lost equals the number gained.
Constructing Cell Notation
Once the half-reactions are balanced, cell notation can be constructed. The anode half-cell is written on the left, followed by a vertical line. The cathode half-cell is written on the right, separated by a salt bridge. The anode is where oxidation occurs, and the cathode is where reduction occurs.
Example Problems
Problem 1:Construct the cell notation for the following redox reaction:
Fe(s) + 2Ag+ (aq) → Fe2+ (aq) + 2Ag(s)
Solution:
- Identify half-reactions:
- Fe(s) → Fe2+ (aq) + 2e- (oxidation)
- 2Ag+ (aq) + 2e- → 2Ag(s) (reduction)
- Balance half-reactions:
- Fe(s) → Fe2+ (aq) + 2e-
- 2Ag+ (aq) + 2e- → 2Ag(s)
- Construct cell notation:
- Fe(s) | Fe2+ (aq) || Ag+ (aq) | Ag(s)
FAQ Summary: Determine The Cell Notation For The Redox Reaction Given Below
What is cell notation?
Cell notation is a concise way to represent an electrochemical cell. It includes information about the anode, cathode, and the overall redox reaction.
How do I identify half-reactions?
Half-reactions are the individual oxidation and reduction reactions that make up a redox reaction. To identify half-reactions, look for changes in oxidation states.
How do I balance half-reactions?
Half-reactions must be balanced in terms of mass and charge. To balance half-reactions, add electrons, protons, and water molecules as needed.